
The branching gives it a tree-like character which is the orgin of the term dendrite.Ĭomputer simulated image of the dendritic solidification of pure nickel. The mechanism of this instability is discussed elsewhere.Ī dendrite tends to branch because the interface instability applies at all points along its growth front. The interface thus becomes unstable and in appropriate circumstances solidification becomesĭendritic. The accumulation of solute and heat ahead of the interface can lead toĬircumstances in which the liquid in front of the solidification front is supercooled. Reichel, Mathematical Modelling of Weld Phenomena III, eds H.
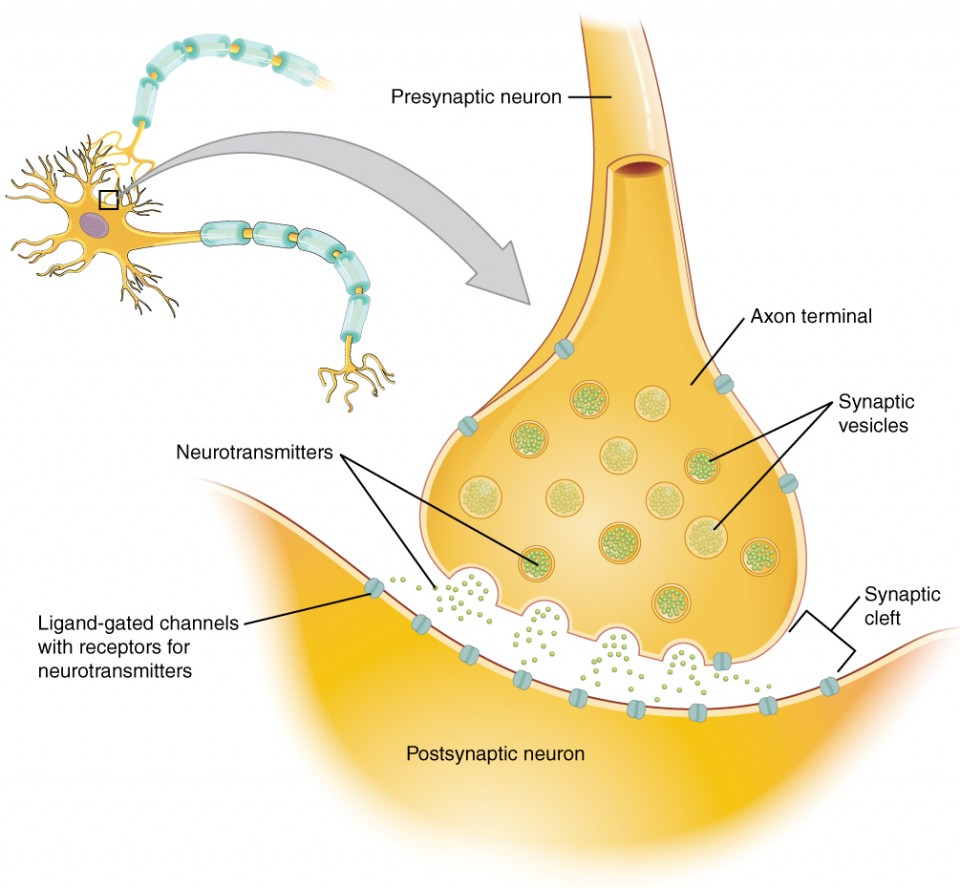
Photograph courtesy of the Institute of Materials, based on the work of U. Similarly, solute may partition into the liquid if its solubility in the solid is less than that in the liquid.Ĭomputer simulated image of dendritic growth using a cellular automata technique. The process may generate heat if the enthalpy of the solid is less than that of the liquid. Once nucleation has occurred, solidification proceeds by the movement of an interface. Alternatively, it may solidify when the pressure is decreased or increased, depending on the sign of the density change. BhadeshiaĪ liquid when cooled solidifies. However, during “fast” charging, the local Li+ concentrations rapidly decrease leading to mass transport limited conditions which result in dendrite growth and lower battery performance.Dendritic Solidification Dendritic Solidification H. During standard charging, the Li+ concentrations at the anode create reaction rate limited conditions that lead to more uniform Li+ deposition. The computational studies demonstrate the mechanisms by which these novel techniques improve the performance of lithium metal batteries such as reducing the pore size in carbon nanomembranes reduces dendrite length and increases deposition density ionic liquid crystal supramolecular assemblies oriented perpendicular to the anode increase the uniformity of Li+ deposition at the anode the effects of homogeneity of ionic conductivity of protective coatings on the anode to enable uniform Li+ deposition.Īdditionally, the model is used to explore how the local conditions in the electrolyte change during battery cycling. Building upon these insights and in collaboration with experimental groups, the effect of the structure of novel coatings and electrolytes on the mass transport to the anode and subsequent dendrite morphology are investigated. Additionally, despite the characterization of battery separators using bulk properties, the heterogeneity of the separators lead to vastly different local transport outcomes. The findings from the simulations suggest that the tortuosity of the separator is a key property affecting transport.
Using SPH, the geometrical parameters of the separator are characterized based on their effect on mass transport and dendrite growth. The first goal is to understand the effects of local transport through battery separators on dendrite growth by explicitly representing commercial battery separator structures taken from SEM images. Using the SPH model, the effect of various structures in the electrolyte on mass transport and dendrite growth are investigated.
Effect of dendrite structure code#
The model is implemented in the LAMMPs code base and includes the ability to model charge/discharge cycles. The SPH model simulates the physics at this interface by solving the governing equations for diffusion, migration, and potential distribution in a binary electrolyte and near a reactive, moving interface and dendrite surfaces. In this dissertation, a meso-scale computational model, using the smoothed particle hydrodynamics (SPH) numerical method, is used to simulate the deposition process at the electrolyte/anode interface of a lithium metal battery.


 0 kommentar(er)
0 kommentar(er)
