
The reader must know the flow of the electrons. Make sure the arrows are clear including the single and half headed arrow. The tail of the arrow begins at the electron source and the head points to where the electron will be. # of valence electrons- (#non bonding electrons + 1/2 #bonding electrons)Ĭurved arrow notation is used in showing the placement of electrons between atoms. Assigning formal charges to an atom is very useful in resonance forms.įormal charge is calculated using this format: An atom with many electrons will have a negative charge. Atoms that are missing one or more electrons will have a positive charge. This is why formal charges are very important.
Meaning of resonance in chemistry plus#
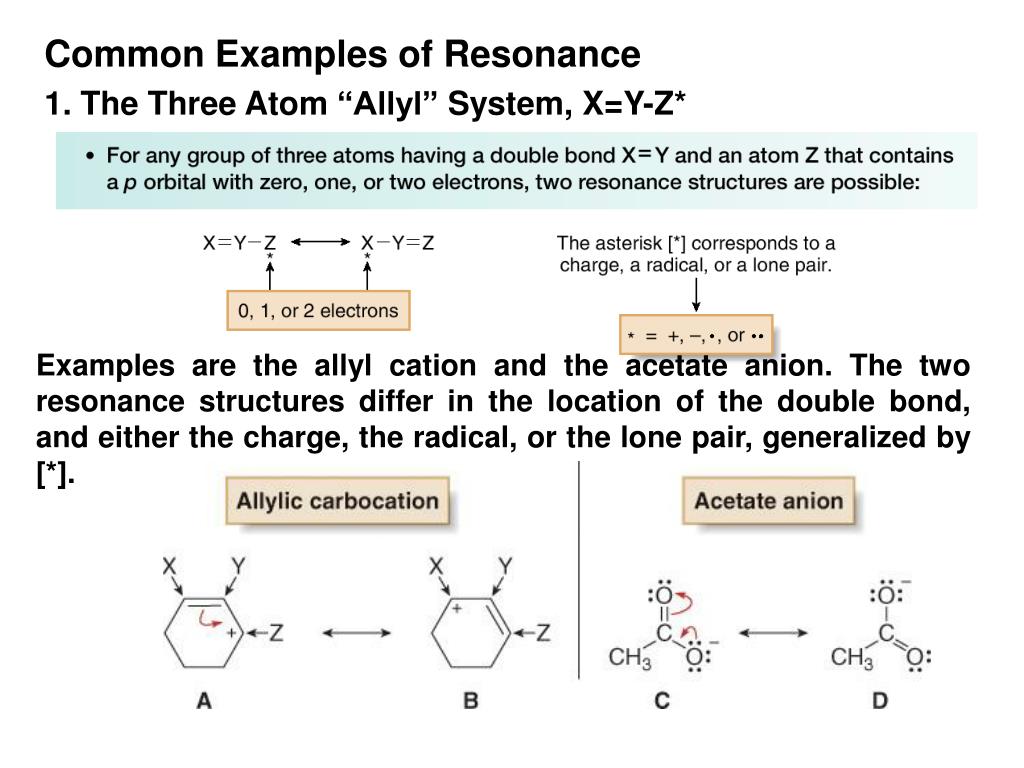
The roots of the word isomer are Greek isos plus meros, or equal parts. Remember, the best resonance structure is the one with the least formal charge. isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same formula) but differ in chemical and physical properties. These structures used curved arrow notation to show the movement of the electrons in one resonance form to the next.įormal charges are used in Chemistry to determine the location of a charge in a molecule and determine how good of a Lewis structure it will be. They are drawn with a double-headed arrow between them to show the actual structure is somewhere between the resonance structures. The more resonance forms a molecule has makes the molecule more stable. The better ones have minimal formal charges, negative formal charges are the most electronegative atoms, and bond is maximized in the structure. Not all resonance structures are equal there are some that are better than others. Resonance structures are a better depiction of a Lewis dot structure because they clearly show bonding in molecules. Resonance forms differ only in arrangement of electrons. The delocalized electron when show movement contributing structures are. Isomers have different arrangement of both atoms and electrons. Resonance is the ability of system to move its pi electrons in the system. Resonance structures are not in equilibrium with each other.

First resonance structures are not real, they just show possible structures for a compound. >Class 11>Chemistry>Chemical Bonding and Molecular Structure>Bond Parameters>Resonance Structures definitionDefine resonanceWhen a molecule is represented by two or more hybrid tructures and that structure are different in the position of electrons not in position of atoms, then the structure is called as resonating structure and this pheno. There are some basic principle on the resonance theory.


 0 kommentar(er)
0 kommentar(er)
